Next Generation Interferon-β
ABN101
Broad Spectrum Prophylaxis
Anti-viral Inhaler therapeutics
Prophylaxis Anti-viral Inhaler Therapeutics ABN101

Summary
ABION is currently developing ABN101, a dry powder inhaler utilizing next-generation interferon-β, as a prophylactic treatment for various respiratory viruses.
The primary objective driving the development of ABN101 is to provide early-stage patients with a preventive measure against severe viral infections, thereby reducing the probability of hospitalization and severity of illness. Additionally, the goal is to stockpile and distribute ABN101 during the initial phases of a pandemic, such as COVID-19, to mitigate casualties before a vaccine becomes widely available.
ABN101 serves as a prophylactic inhaler, meticulously tailored to offer preventative care for at-risk individuals vulnerable to moderate or severe viral infections. Harnessing the heightened stability of interferon-β, ABN101 triggers the innate immune response within cells, prompting them to activate the adaptive immune response. This dual immune mechanism provides robust protection against diverse respiratory viruses. Administering ABN101 preemptively during the early stages of viral infection proves highly effective, capitalizing on the body’s innate immune response to combat various respiratory virus strains.
Traditional cytokines present considerable bioprocessing challenges due to their inherent instability. However, ABION’s groundbreaking advancement with ABN101 has significantly bolstered its stability, leading to heightened productivity. This newfound stability has facilitated the development of diverse ABN101 formulations, including a dry-powder variant. This dry-powder formulation can be seamlessly integrated with commonly used inhalers, particularly those utilized by asthma patients. Offering benefits such as straightforward storage, portability, and user-friendly administration, this dry-powder inhaler emerges as a highly accessible medical solution, particularly during pandemics or outbreaks of respiratory viruses.
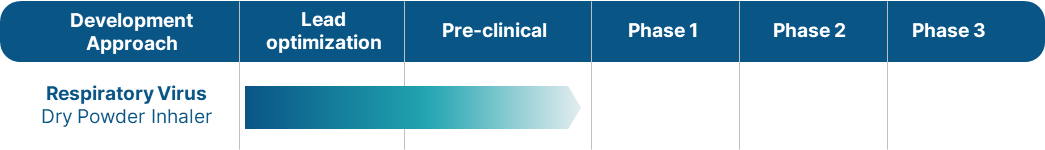
ABN101 has demonstrated its effectiveness in inhibiting various COVID-19 variants and other viruses in cell lines. Presently, ABN101 is undergoing preclinical evaluation in animal models using an inhaler delivery system. Encouraged by the promising preclinical outcomes, we anticipate submitting an Investigational New Drug (IND) application in 2025, marking a significant step forward in ABN101’s progression towards clinical trials.
Mechanism of Action
ABN101 is a biopharmaceutical that utilizes both the innate and adaptive immune responses of cells
to effectively suppress respiratory viruses in their early stages, thereby offering a preventive therapeutic effect.
What is Interferon?
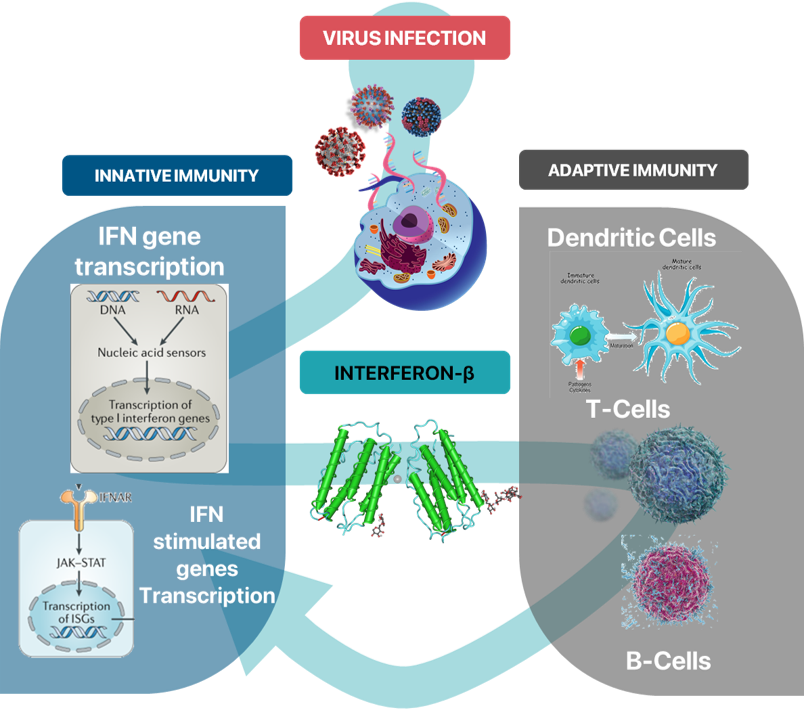
Interferon, a protein produced by immune cells, is part of the cytokine family responsible for orchestrating immune responses and signaling during foreign invasions. Type 1 interferons, such as interferon-alpha and interferon-beta, are frequently utilized as biopharmaceuticals. Interferon triggers innate immune responses within cells, hindering viral replication, and also prompts adaptive immune responses involving natural killer cells (NK cells), dendritic cells, T cells, and B cells to combat the infection.
Interferon-beta, produced by most viruses through this mechanism, has demonstrated significant promise as a preventive treatment when administered promptly after infection or at the early onset of symptoms. During the initial stages of the COVID-19 pandemic in 2019, it was prescribed as an off-label medication in the United States to alleviate symptoms in patients awaiting a definitive cure.
However, bioprocessing natural interferon substances presents considerable challenges, resulting in high production costs that impede widespread administration, especially during the early phases of viral infections. Additionally, the limited formulation options restrict their suitability for non-hospitalized patients.
With Enhanced Stability,
Bringing Care Closer to Patients
ABN101, a dry powdered inhaler, represents an innovative solution for preventing and treating severe respiratory viruses across various types. This prophylactic inhaler treatment for respiratory viruses overcomes the limitations associated with conventional interferons. By harnessing the enhanced stability of second-generation interferon-beta, we’ve achieved remarkable productivity.
Additionally, ABN101’s formulation into a powdered form capitalizes on its exceptional stability profile. Integrated with an inhaler, a familiar device among individuals with bronchial or lung diseases, it ensures precise administration to the respiratory virus infection pathway. This approach not only offers convenience but also maximizes therapeutic efficacy for patients.
Administering ABN101 via an inhaler, either before or immediately after viral infection, enables direct delivery to the bronchi and respiratory tract, the primary sites of viral replication. This prompts a swift immune response, aiding in the elimination of the virus and averting the onset of severe illness. With these unique attributes, our company is committed to advancing ABN101 as a prophylactic drug, an area ripe for further exploration and development.
Research & Development

Development phase
ABN101
Pioneering Prophylactic Inhaler Therapy for a Broad Spectrum of Respiratory Viruses.
Development Strategy
ABN101, which can be used for various types of respiratory viruses,
is a medication that can be tailored to fit the strategies of various partners.



