– [ESMO] “Application of liquid biopsy and AI in clinical trials for ‘vabametkib + lazertinib’ combination therapy”
– “Rapidly worsening prognosis in c-MET overexpressing lung cancer patients highlights the importance of rapid clinical trials”
– “AI enhances subject selection for clinical trials, boosting accuracy”
ABION Vice President Choi Jun-young giving an explanation at the abstract presentation venue during the recent 2024 European Society for Medical Oncology (ESMO) congress held in Barcelona, Spain.
ABION Vice President Choi Jun-young giving an explanation at the abstract presentation venue during the recent 2024 European Society for Medical Oncology (ESMO) congress held in Barcelona, Spain.
[by Lee, Young Sung] ABION, a Korean novel drug development company, is combining liquid biopsy and artificial intelligence (AI) technology to improve the clinical effectiveness of its small molecule combination therapy using ‘vabametkib’ and ‘lazertinib’. The aim is to quickly select appropriate clinical candidates through liquid biopsy and increase success rates by leveraging AI to increase accuracy.
Lazertinib is a non-small cell lung cancer (NSCLC) treatment developed by Yuhan Corporation. Recently, Janssen, a subsidiary of the multinational pharmaceutical company Johnson & Johnson (J&J), obtained approval from the U.S. Food and Drug Administration (FDA) for its combination therapy of ‘Rybrevant’ and ‘lazertinib’ as a first-line treatment for NSCLC. Janssen is supplying lazertinib for ABION’s combination therapy clinical trial.
EGFR tyrosin kinase inhibitors (TKIs), such as Tagrisso (osimertinib) and lazertinib are not exempt from resistance issues. This resistance is primarily attributed to the Mesenchymal Epithelial Transition (MET) mutations. Therefore, c-MET inhibitors are anticipated to have a synergistic effect when used in combination with EGFR TKIs. The MET gene is also known as c-MET or hepatocyte growth factor receptor (HGFR).
Normally, c-MET is involved in cell growth and survival, but mutations in the c-MET gene lead to abnormal cell growth, causing cancer. In other words, when activated, c-MET drives the proliferation and division of cancer cells. Vabametkib binds to the tyrosin kinase (TK) domain in cancer cells, blocking c-MET signals to halt this process.
ABION’s strategy involves using liquid biopsy to quickly identify patients with ‘c-MET CTC (circulating tumor cells positive for hepatocyte growth factor receptor)’ before the clinical trial of the ‘vabametkib + lazertinib’ combination therapy. These patients are then registered and proceed with the clinical trial. CTCs arise due to overexpression of c-MET.
In an interview with at the 2024 European Society for Medical Oncology 2024 (ESMO) congress held in Barcelona, Spain, ABION Vice President Choi Jun-young stated, “Our analysis of CTCs with overexpressed c-MET revealed a correlation with resistance.” He elaborated, “Specifically, lung cancer patients exhibiting c-MET overexpression experience a rapid deterioration in prognosis. By swiftly identifying these patients as clinical subjects, we can enhance treatment effectiveness and improve clinical outcomes, which is why we started this research.”
“In this context, liquid biopsy serves as a tool for rapidly identifying suitable subjects, thereby improving the effectiveness of the vabametkib and lazertinib combination study,” Choi further expressed.
Choi also explained that the toxicity issues associated with c-MET, which was identified as a reason for the lack of success of the ‘EFGR TKI + c-MET treatment’ combination therapy in the global market, has been addressed with vabametkib. “At present, vabametkib offers the safety profile for c-MET treatment,” Choi emphasized.
In addition, Abion plans to leverage artificial intelligence (AI) technology to precisely identify patients with c-MET CTC overexpression.
“Whereas the degree of c-MET overexpression was previously determined based on the doctors’ intuition, we will now use AI to more precisely define the range and select only overexpressed subjects,” Choi explained. “Relying solely on AI poses certain risks, so we will combine traditional methods with AI-assisted approaches to increase the chances of clinical success.”
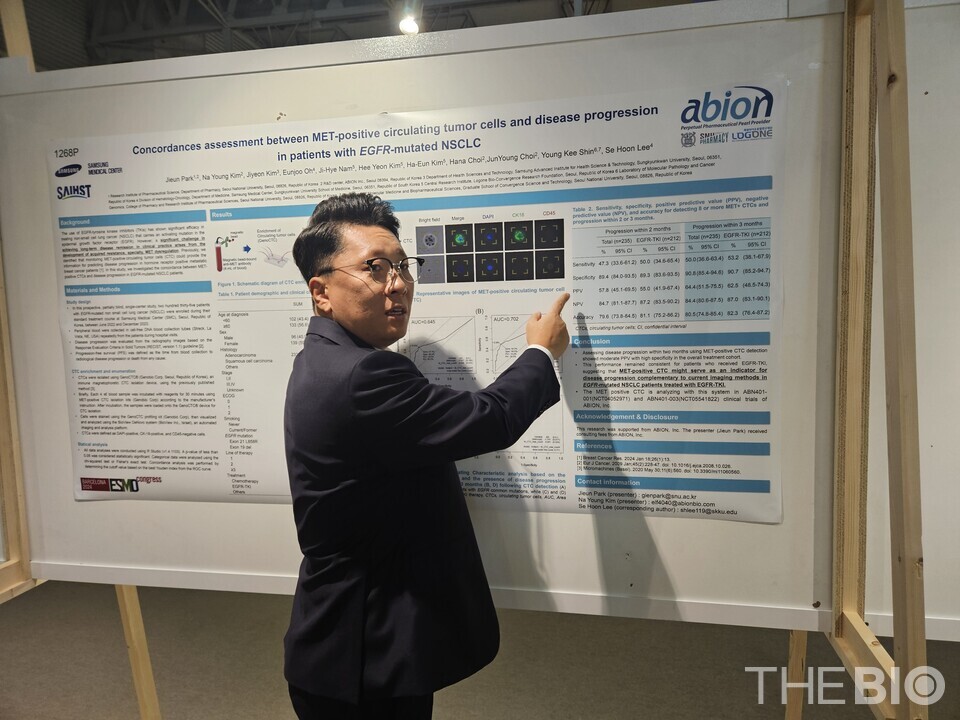
Abion presented the abstract of the related liquid biopsy study as a poster at this year’s ESMO congress. The study focused on c-MET CTC disease progression in patients with EGFR mutation-positive non-small cell lung cancer (NSCLC) who had undergone EGFR TKI treatment. It was conducted as an investigator-led clinical trial (IIT) by Professor Lee Se-hoon’s research team from Samsung Medical Center.
An analysis of disease progression over two months in 212 patients who received EGFR TKI treatment showed a sensitivity of 50%, specificity of 89.3%, positive predictive value of 55%, negative predictive value of 87.2%, and accuracy of 81.1% in patients with eight or more MET CTC. The company stated that these findings suggest MET CTC could serve as a complementary indicator alongside currently imaging methods.
“This study shows that patients with c-MET mutations can be identified early, allowing for timely and appropriate treatment,” Choi remarked. “In the clinical trial of the vabametkib and lazertinib combination therapy, starting treatment at the appropriate time through a pre-examination process using c-MET CTC can increase the chances of clinical success.”
The vabametkib combination clinical trial will begin with 18 subjects in the first phase, followed by 60 subjects in the second phase, and 80 subjects in the third phase, with a total of up to 158 subjects. Currently, vabametkib is also undergoing a global phase 2 clinical trial as a monotherapy (cohort 1), involving 40 subjects across the United States, Korea, and Taiwan.
Published by: http://www.thebionews.net/news/articleView.html?idxno=9145