C-MET Tyrosine Kinase Inhibitor
ABN401
ABN401 Best-In-Class C-MET TKI For NSCLC Patient
“BEST-In-CLASS c-MET TKI” ABN401

Summary
ABN401 is an innovative tyrosine kinase inhibitor specifically designed to target c-Met mutant cancers.
The c-Met protein plays a crucial role in cancer cell growth and angiogenesis, making it an attractive target for therapeutic intervention. Mutations in c-Met have been observed in various cancer types, and recent research has highlighted the poor prognosis associated with these mutations. Notably, MET Exon 14 skipping mutations have been reported in approximately 3% of patients with non-small cell lung cancer (NSCLC). Moreover, recent research demonstrates that c-Met mutations are the predominant mechanisms of resistance to EGFR TKIs.
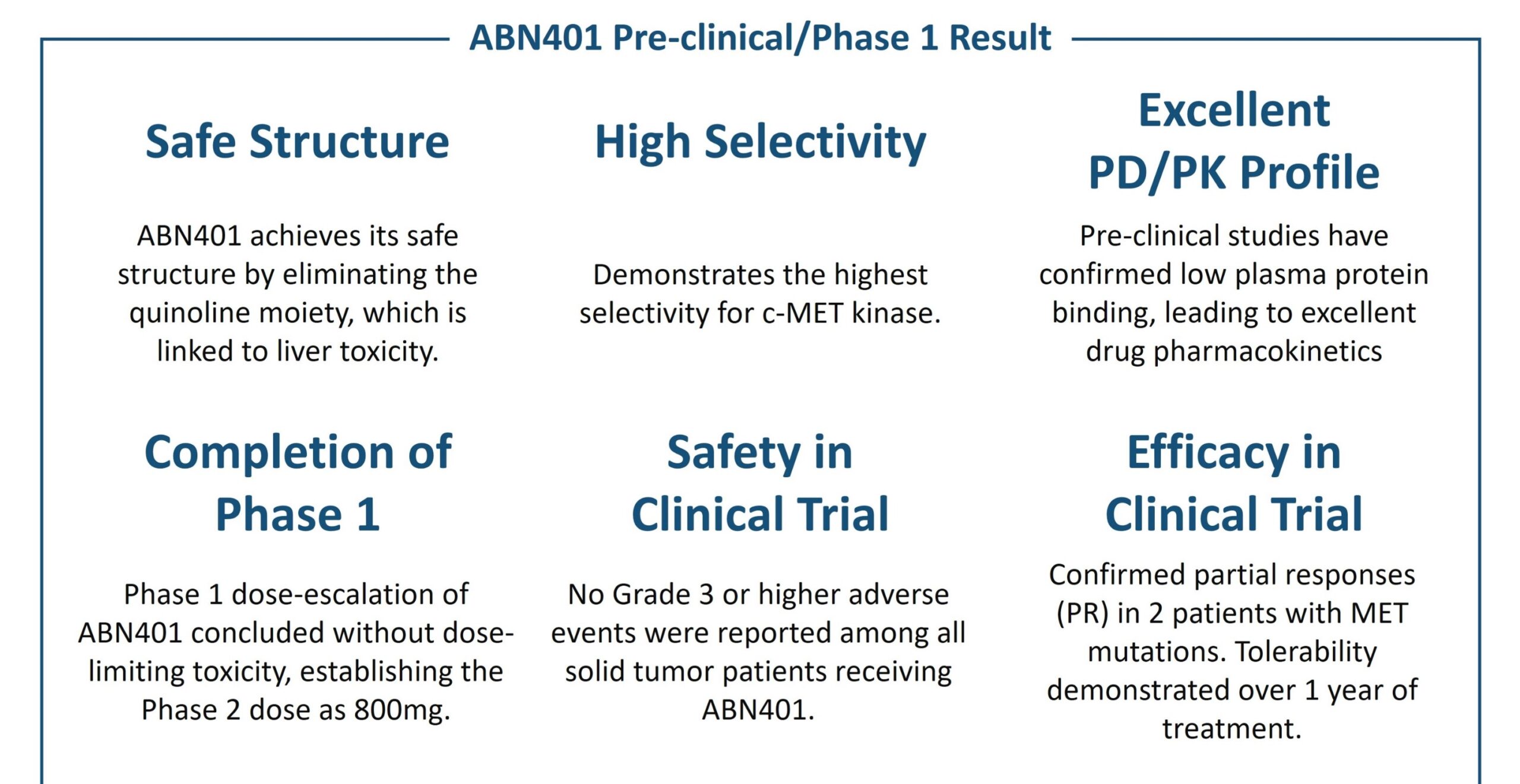
In Phase 1 clinical trials, ABN401 has demonstrated exceptional safety profiles. During a dose-escalation study, no grade 3 or higher adverse events were reported, indicating a favorable safety profile. Additionally, promising efficacy outcomes were observed, with two partial response noted during the Phase 1 trial. Building upon these encouraging results, ABN401 is currently advancing through Phase 2 clinical development, focusing on patients with MET exon 14 deletions in NSCLC. The objective is to provide a targeted therapy option that prioritizes patient safety.
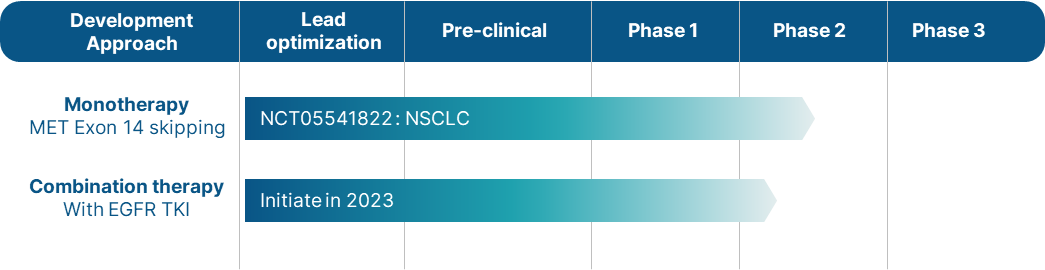
ABION announced in January 2023 that the first patient had been enrolled in the Phase 2 trial for ABN401. This trial is currently ongoing and is registered under the National Clinical Trials (NCT05541822) database. By evaluating the efficacy and safety of ABN401 in a larger patient population, this Phase 2 trial aims to further validate its therapeutic potential and bring us closer to offering an effective treatment option for NSCLC patients with MET exon 14 skipping mutation.
Mechanism of Action
ABN401
binds to the ATP binding site of the MET tyrosine kinase and inhibits the kinase activity.
ABN401 targets MET mutation in NSCLC.
ABN401 represents a precision oncology medicine targeting c-MET, a key player implicated in the development of various solid tumors.
c-MET, also referred to as Hepatocyte Growth Factor Receptor, is a tyrosine kinase receptor that becomes activated upon binding with hepatocyte growth factor (HGF). The c-MET protein comprises two distinct regions: one that receives signals from HGF outside the cell and another that converts these signals into downstream intracellular responses. Due to its involvement in cell growth and angiogenesis, any mutations in c-MET can lead to a higher susceptibility to cancerous transformation, with cancer cells often exhibiting overexpression of c-MET or abnormal growth due to disrupted signaling.
Notably, three types of c-MET mutations have been identified in non-small cell lung cancer (NSCLC): MET Exon 14 skipping, characterized by the deletion of the regulatory region in exon 14, resulting in persistent signaling; MET Amplification, involving the amplification of the MET gene and subsequent dysregulation of signaling; and MET Overexpression, where the MET protein is excessively expressed, leading to signaling irregularities.
ABN401 functions as a targeted anti-cancer agent by precisely obstructing signal transduction. By binding to the ATP-binding sites and inhibiting phosphorylation of downstream signaling, ABN401 effectively disrupts the aberrant signaling cascade. Importantly, it has also demonstrated efficacy in MET mutant cancer cells that are not reliant on HGF for activation.
Our overarching objective is to develop ABN401 as a treatment option for a range of solid tumors, initially focusing on non-small cell lung cancer due to its high incidence and mortality rates. Subsequently, we intend to expand its indications to include gastric and liver cancers, among others, as part of our comprehensive strategy for combating these challenging diseases.
Research & Development
Clinical Trial
ABN401
ABN401 is a c-MET tyrosine kinase inhibitor targeting c-Met mutant cancers, currently in a global Phase 2 study for patients with MET exon 14 skipping non-small cell lung cancer (NCT05541822).
For Patients and Caregivers
At ABION, we are deeply dedicated to developing precision oncology therapeutics to improve the lives of individuals affected by cancer and to provide them with better treatment options. Whether you’re a patient, family member, caregiver, or part of a support group, we understand the challenges you face and are here to offer support and guidance.
For clinical information, please visit our webpage (▶ABN401 Clinical Trial]). Additionally, details about hospitals and clinicians participating in clinical trials can be found in the ‘Contacts and Locations’ section on our website.
Warm regards,
ABION Team


